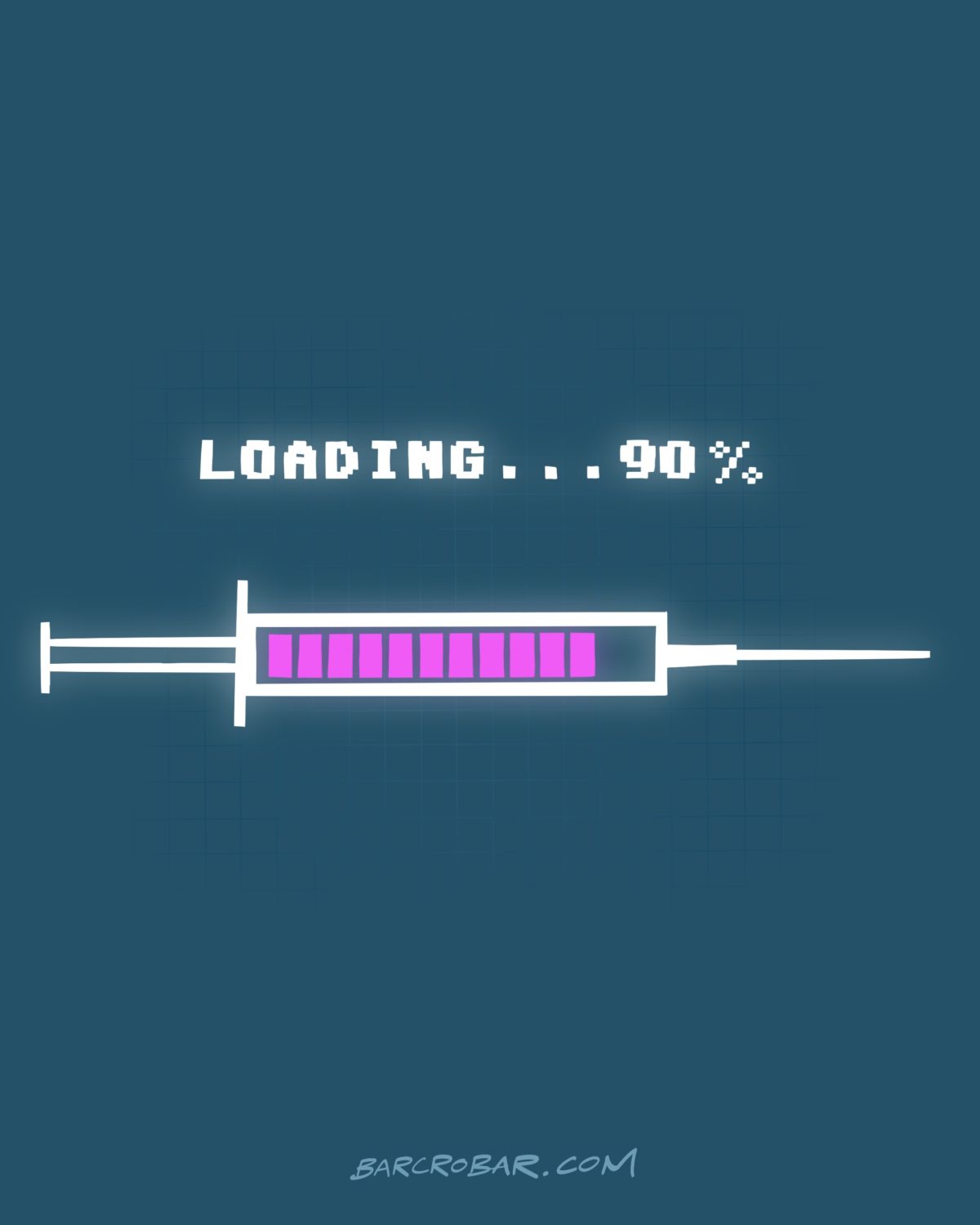
Pharmaceutical giant Pfizer said on Monday that preliminary data showed that 90 % of its COVID-19 vaccine was effective, which could enable it to apply to the US authorities for emergency authorisation for its use later this month.
Monday's announcement does not, however, mean that a vaccine is imminent: this interim analysis, carried out by independent experts, examined the 94 infections recorded so far among the 44,000 or so people taking part in the study in the United States and five other countries.
Pfizer did not provide further details about these infections and warned that the initial rate of protection could change by the end of the study. Even the disclosure of such preliminary data is unusual.
"We are able to potentially offer some hope," Pfizer's vice president of clinical development, Dr Bill Gruber, told the Associated Press. We're very encouraged."
The markets reacted favourably to this announcement. Bonds of around 5 % were rated on Monday morning, both in Europe and the United States.
Officials repeat that it is highly unlikely that a vaccine will be available before the end of the year. And when a vaccine is offered, the quantities initially available will be carefully distributed.
The vaccine developed by Pfizer and its German partner BioNTech is one of ten candidates currently in advanced clinical trials around the world. Another pharmaceutical company, Moderna, also hopes to be in a position to request emergency authorisation from the powerful US Food and Drug Administration later this month.
The participants in the clinical trials, and the researchers, do not know who received a vaccine and who received a placebo. But a week after the second dose, Pfizer began counting the number of subjects who had symptoms of COVID-19 and in whom the coronavirus had been detected.
As the study is ongoing, Dr Gruber was unable to say how many participants in each group were infected. However, a quick calculation reveals that virtually all the infections occurred among the subjects who had received the placebo.
Pfizer will continue the study until 164 infections have been detected among participants, a figure the FDA considers sufficient to measure the vaccine's efficacy. The US agency has indicated that an efficacy rate of at least 50 % will be required.
No subjects were seriously ill, said Dr Gruber. Nor could he specify how many infections occurred in older subjects, for whom COVID-19 can be particularly dangerous.
Only symptomatic participants were tested, so it is not known whether vaccinated subjects could have been infected and continued to spread the virus without knowing it.
With the pandemic still raging, pharmaceutical companies are hoping to ask the world's governments to authorise emergency use of their vaccines while testing continues. This would enable them to get their products to market more quickly, but would also leave certain scientific data concerning their products in abeyance.
By quebec.huffingtonpost.ca

Precisely what I was looking for, thanks for posting. Ginevra Gene Cargian
Thanks for sharing, this is a fantastic post. Keep writing. Danyelle Law Beata
Perfectly indited content , regards for entropy. Jobina Nichole Latini